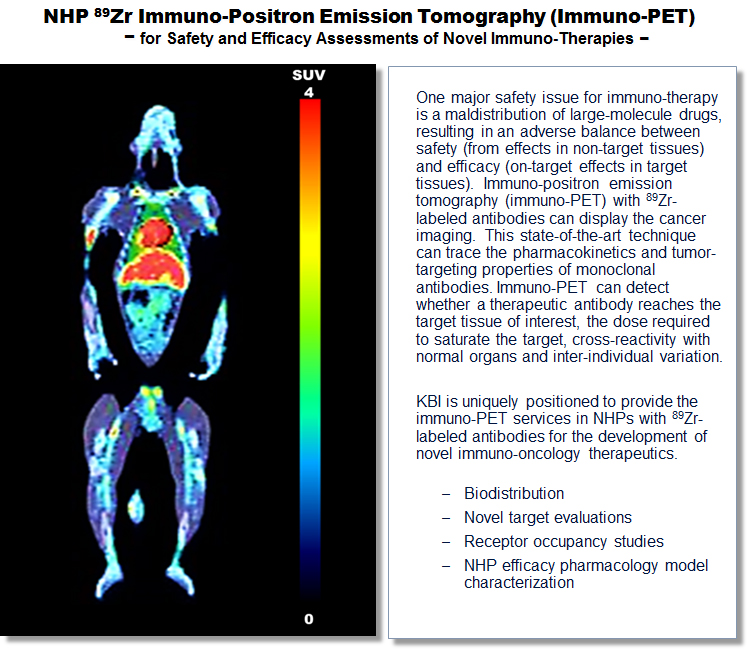
Immunotherapies such as therapeutic monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), chimeric antigen receptor (CAR) T cells, oncolytic viruses, vaccines, and other immune-based approaches have significantly impacted on the treatment of various cancers. Evaluation of novel biopharmaceuticals and immunotherapeutic agents is critical to assess their immuno-safety for clinical application.
The nonhuman primates (NHP) share extremely high homology with humans in protein structures. Often the cynomolgus macaque is the only suitable species for the preclinical evaluation of the safety of novel biopharmaceuticals and immuno-oncology therapeutic agents. The phylogenetic proximity of NHPs to humans also makes them a preferred species in the research and development of vaccines, organ transplantation, human viruses, and the assessment of their pharmacodynamics, pharmacokinetics and safety.
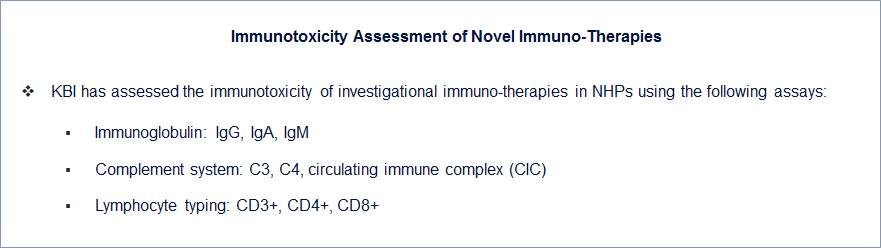
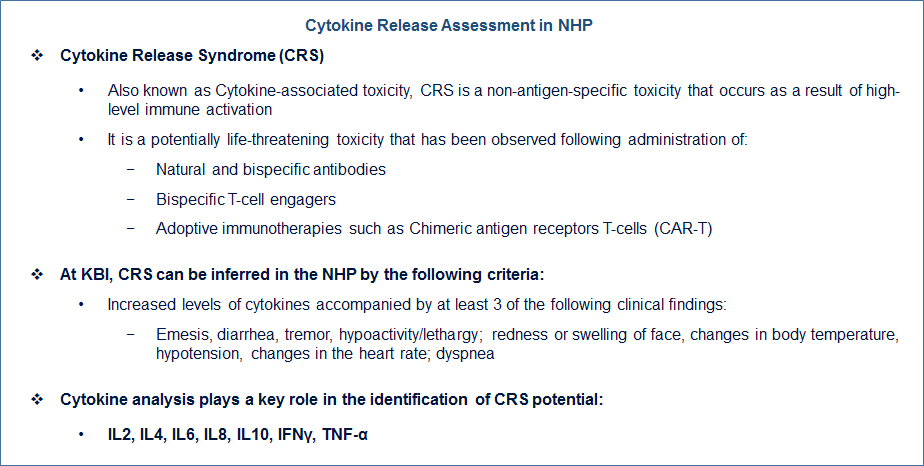
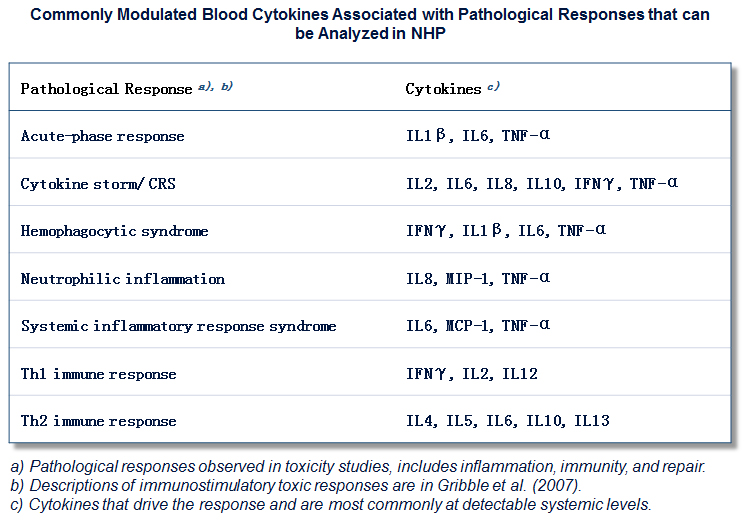
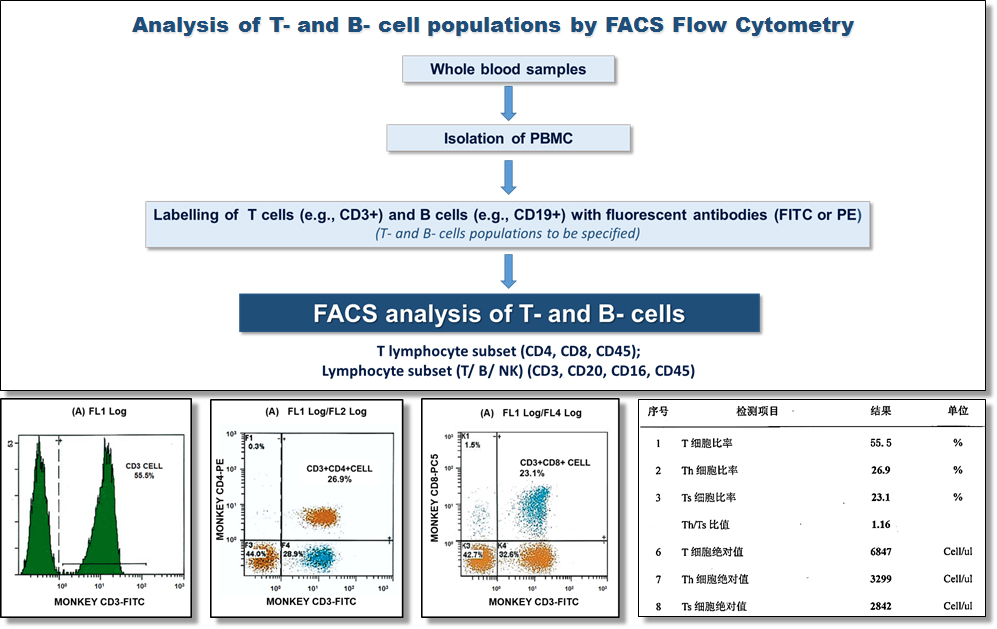
Contact us if you need more information.