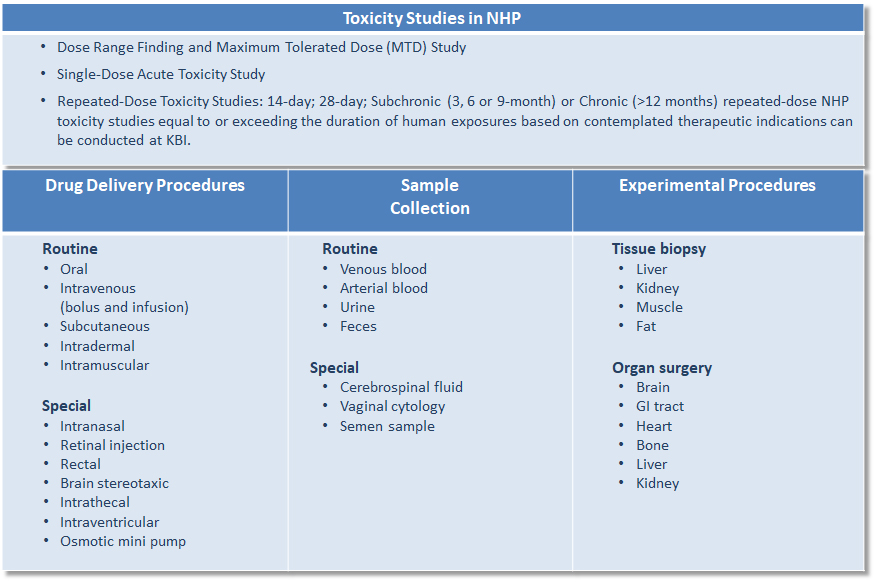
KBI's Safety Assessment Program can be incorporated into our In vivo Pharmacology / Efficacy Evaluation study designs and protocols or offered as a stand-alone package. We offer customized toxicity studies that can be performed in healthy nonhuman primates (NHP), as well as, in our cohort of obese, dysmetabolic NHPs or in any of our validated translatable disease models. We work closely with our sponsors to understand and match their requirements and ultimate research goals.
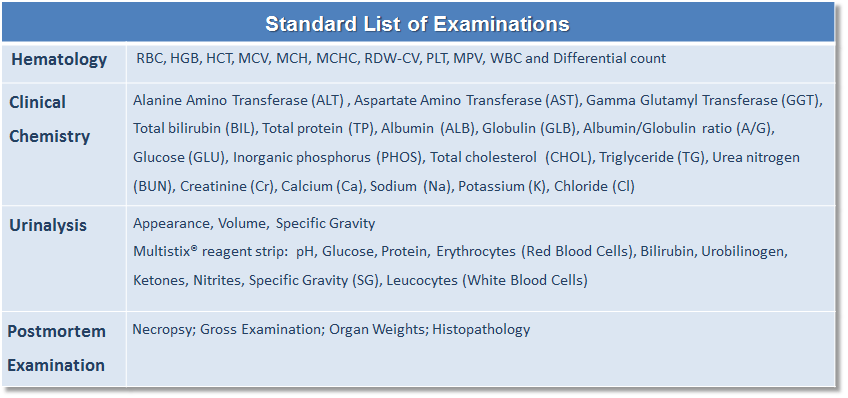
The KBI Safety Assessment Portfolio is an extensive platform of preclinical services which includes, but may not necessarily be limited to: Protocol design/preparation/writing; IACUC review; in-life study conduct; biological sample collection and clinical pathology analysis; statistical data evaluation/analysis; TK profiling; postmortem necropsy, gross examination, organ weights and histology, histopathological slides preparation, special staining, immunohistochemistry; and, study report and document preparations to assist the sponsor for IND filing.
Studies are conducted by highly professional Study Directors with proven track record in global CROs and extensive background/experience in a GLP regulatory work environment. Our SDs work with dedicated and reliable teams of scientific researchers and technical support staff from reputable medical/ science-oriented universities and prestigious academic research institutions.
KBI’s state-of-the-art research facilities have been custom-designed based on ideal operating concepts adapted from some of the world’s best preclinical facilities which include separation of work areas, environmental control monitoring and management, and high-level access security systems. The 24-hour continuous operations are maintained by Facility operations staff who are highly experienced in CRO facility maintenance management.
Contact us if you need more information.