Kunming, China, June 29, 2023
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in humans closely associated with central obesity, dyslipidemia, insulin resistance and diabetes. This disease starts from simple steatosis and can progress through nonalcoholic steatohepatitis (NASH) to liver fibrosis, cirrhosis and hepatocyte carcinoma.
KBI has developed the monkey models of NAFLD and NASH with typical pathological hallmarks as seen in humans by feeding the KBI designed high fat diet over 2 years. The monkeys are first screened for obesity, dyslipidemia, insulin resistance, diabetes and hypertension to characterize the stage of their NAFLD (Fig 1). KBI can provide NASH animals at different grades and stages for different needs and designs by using the state-of-the-art technologies including ultrasound, EXDA (shown in platform page), histopathology, biomarkers (Fig 2) and MRI (Figs 3 and 4).
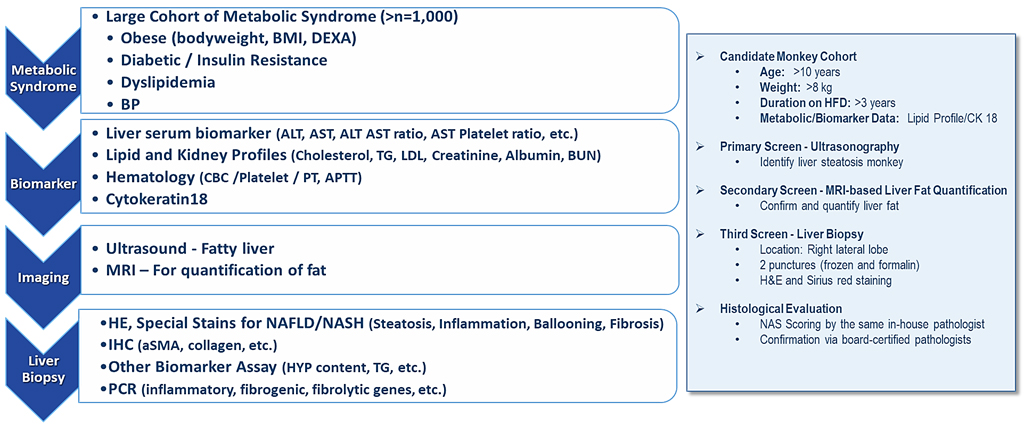
Fig 1. Screening process for NAFLD and NASH. Based on the NASH Clinical Research Network (Hepatology 2011,53:810-820). The monkeys with in 2 or more metabolic dysfunctions are then selected for liver biopsy to examine histopathological lesion and fibrosis.
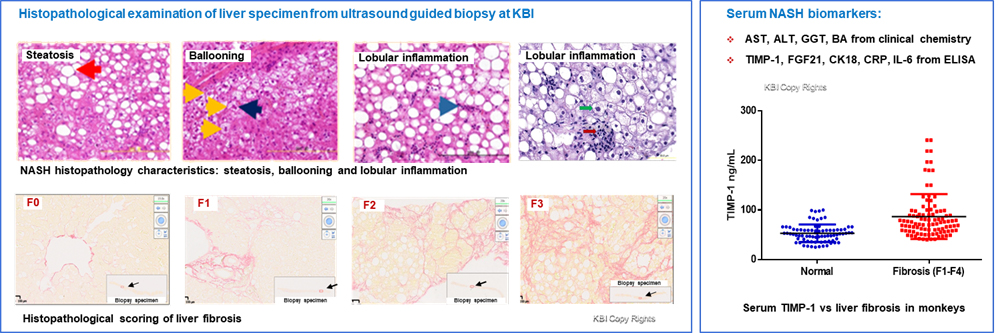
Fig 2. Histopathology of liver and serum biomarkers from NAFLD/NASH monkeys. The histopathological characteristics from liver biopsy in monkeys (Left) at KBI shows typical NASH characteristics as defined in humans (N Engl J Med 2017,377:2063-2072). KBI also has a battery of serum biomarkers to monitor the progression of NASH and liver fibrosis (Right).
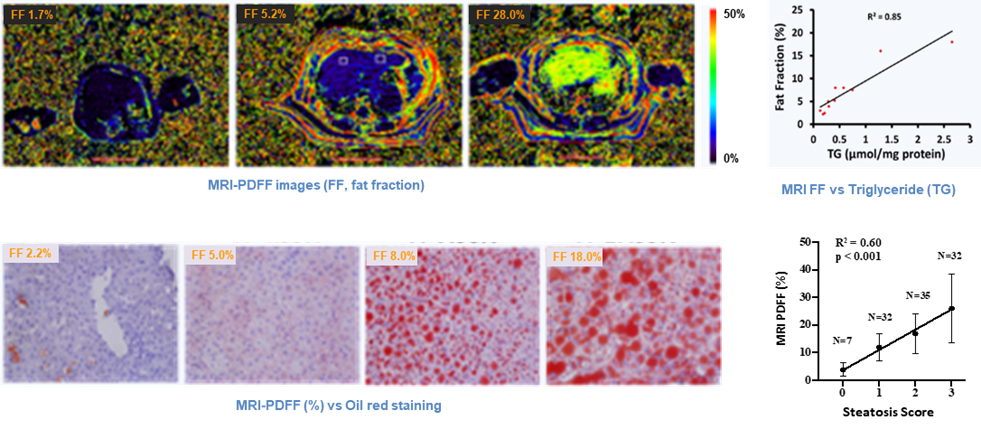
Fig 3. Quantification of liver steatosis by MRI PDFF vs enzymatic assay, oil red staining and pathological scoring in monkeys.
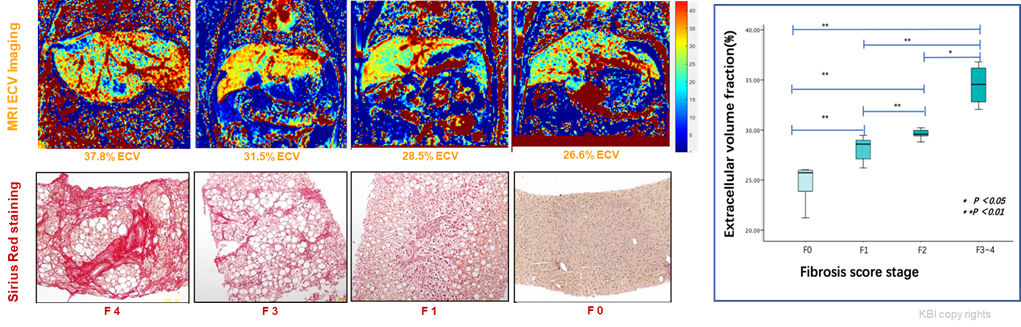
Fig 4. MRI extracellular volume (ECV) for the assessment of liver fibrosis vs histopathological fibrosis scoring.
Contact us if you need more information.